Creatine kinase
Creatine kinase (CK, EC 2.7.3.2.) is the central regulatory enzyme of energy metabolism. The enzyme catalyzes the transfer of a phosphoryl group from creatine phosphate to ADP, thereby forming creatine and ATP:
Creatine phosphate + ADP ↔ Creatine + ATP
Thus, CK connects sites of ATP production (glycolysis and mitochondrial oxidative phosphorylation) with subcellular sites of ATP utilization where it rapidly regenerates ATP in situ from phosphocreatine near these ATPases. While low CK activities associated with organ hypofunction have been thoroughly studied, recent research lines focus on high CK activity implied in hypertension, bleeding risk and cardiovascular disease.
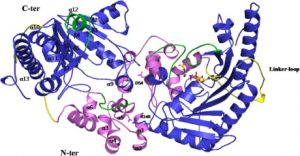
A diagram of the hBB-CK dimer is shown. The overall structure of hBB-CK consists of a smaller N-terminal domain (violet area) and a larger C-terminal domain (blue area). The ligands are depicted as sticks, and the Mg2+ ion is depicted as a ball. The linker loop is depicted in yellow, and the two loops of the active site are in green. The monomer–monomer interface residues are D54 and R148.
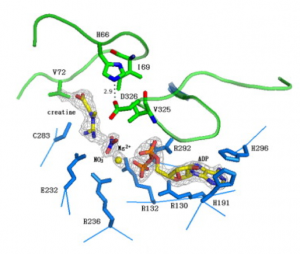
This figure depicts the active site residues of the hBB-CK transition-state-analogue complex; TSAC. The ligands are depicted as stick models. The ligand atoms, ADP–Mg2+–creatine and nitrate (NO3−) are shown in yellow and green, respectively, and the protein residues are shown in blue. The gray mesh seen at the contour level represents 4σ (NO3−, Mg2+), 1.5σ (creatine) and 3σ (ADP) in a 2Fo–Fc electron density map.
Reference S.M. Bong et al. / FEBS Letters 582 (2008) 3959–3965
Current frontiers in creatine kinase research
. Creatine kinase and blood pressure


